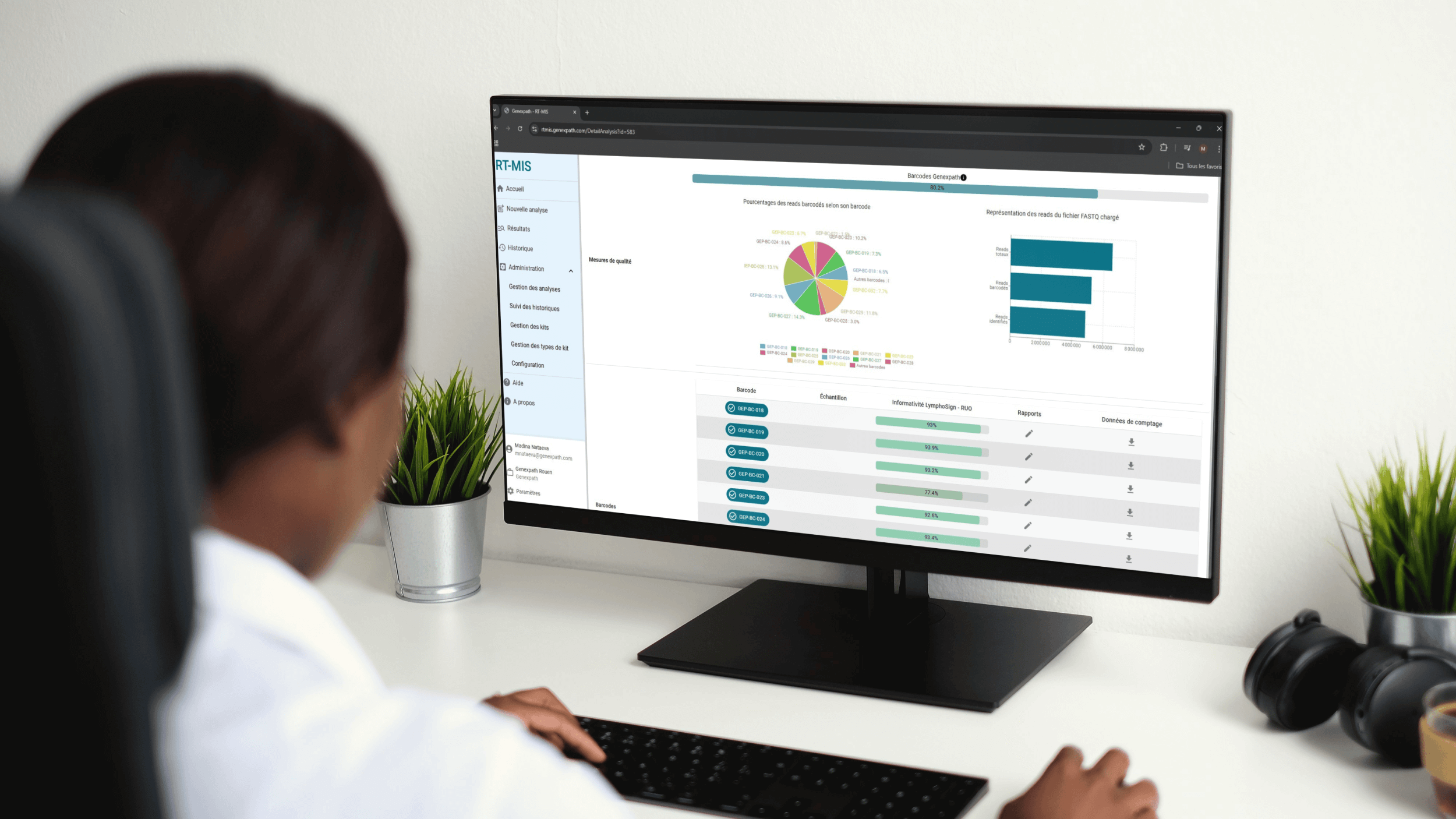
The in-depth understanding of non-Hodgkin lymphoma (NHL) has become a major challenge, and many advances have been made since the introduction of the WHO classification in 2001.
However, the risk of diagnostic errors remains high due to the great diversity of lymphomas. There are in fact more than 80 subtypes of non-Hodgkin lymphoma, some of which are rare and particularly complex to characterize.
The LymphoSign test However, the risk of diagnostic errors remains high due to the great diversity of lymphomas. There are in fact more than 80 subtypes of non-Hodgkin lymphoma, some of which are rare and particularly complex to characterize.
This test is marked CE-IVD for diagnostic use in Vitro test, guaranteeing its compliance with European safety and performance requirements.
This test is suitable for classifying the following subgroups:
- High-grade non-Hodgkin lymphoma type B
- DLBCL ABC
- DLBCL GCB
- DLBCL PMBL
- Low-grade non-Hodgkin lymphoma type B
- Follicular lymphoma (FL)
- Mantle cell lymphoma (MCL)
- Marginal zone lymphoma (MZL)
- Lymphocytic lymphoma (SLL)
- Type T non-Hodgkin lymphomas
- Th angioimmunoblastic T-cell lymphoma (AITL)
- ALK-positive anaplastic large cell lymphoma (ALCL ALK+)
- ALK-negative cytotoxic anaplastic large cell lymphoma (ALCL-ALK-cytotox)
- ALK-negative Th2 anaplastic large cell lymphoma (ALCL-Th2 ALCL)
- Adult T-cell leukemia-lymphoma (ATLL)
- NK/T cell lymphomas (NKTCL)
LymphoSign test characterizes an innovative, precise and reliable test for pathologists and molecular biologists. This test is used to characterize non-Hodgkin's lymphoma (NHL).
It evaluates the degree of differentiation of tumor cells by analyzing the expression level of more than 130 relevant genetic markers, based on ligation-dependent PCR (RT-MLPSeq) technology.
The simplicity of the test makes it possible to obtain the results of the analysis from tumor RNA within 24 hours. Additionally, the protocol is optimized for low quality samples like FFPE samples.
Thanks to artificial intelligence trained on a base of more than 3000 cases, the RT-MIS platform establishes the most probable classification between 13 subtypes of NHL B and T.
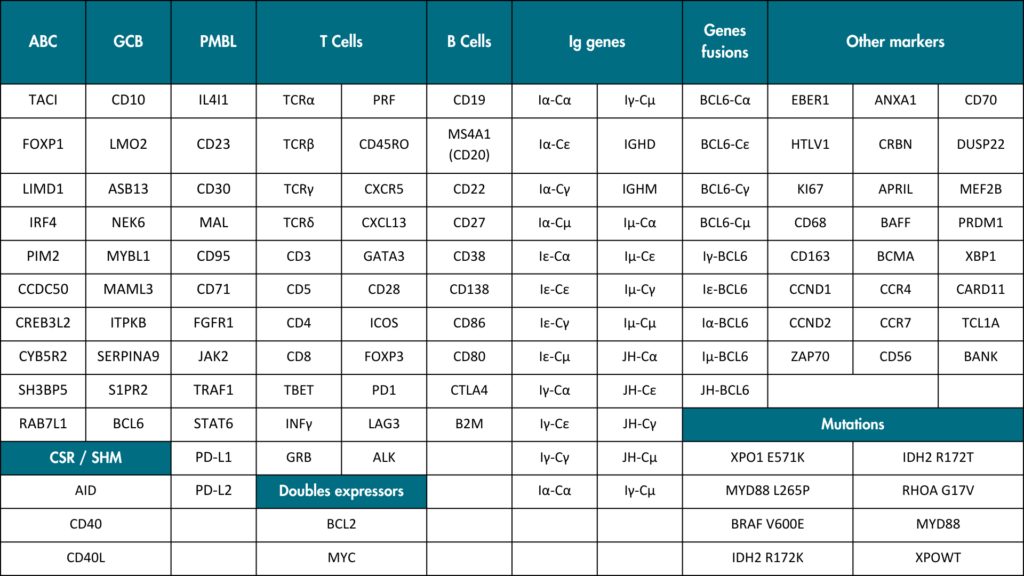
List of LymphoSign test markers
All 137 markers included in the test are listed and arranged by groups. The panel includes B cell genes, immunoglobulin genes, T cell genes, detection of recurrent somatic mutations, so-called “double expressor” genes, ABC discriminant genes are labeled in blue, GCB genes in orange, PMBL genes in red.
The key points of the protocol
In Vitro test
- 4 big steps
- 1/2 day including 1 hour to 1 hour 30 minutes of actual handling
- Sensitive thanks to short probes
- Optimized for FFPE samples
Sequencing
- Adapted to Illumina technology
- Possible to Sequencing with others
- Only 100,000 reads are needed per sample
Analyzed
- Increased specificity thanks to UMI
- Bioinformatics analyzes included
- Access to all raw data
| Cancer type | Non-Hodgkin's lymphomas B or T |
| Application domain | Gene expression |
| handling duration | ≃4h (without sequencing) |
| Actual working time | ≃1h-1h30 (without sequencing) |
| Sample types | Fresh, frozen or fixed and paraffin-embedded tissue biopsies |
| Input quantity | Between 50 and 500 ng of ARN in a volume of 2µL |
| Reagents | Probes targeting 137 markers of interest, barcodes, sequence primer |
| Material compatibility | Sequencer Illumina® |
| Bio informatique analyses | Access to the RT-MIS analysis platform included |
| Accessible data | Classification report, analysis graphs and complete raw data (csv) |
Kit composition
What the kit contains:
- RT-MLPA probe mix
- Genexpath barcodes
- Sequencing primers
Available in 8, 16, 24 or 48 analyzes
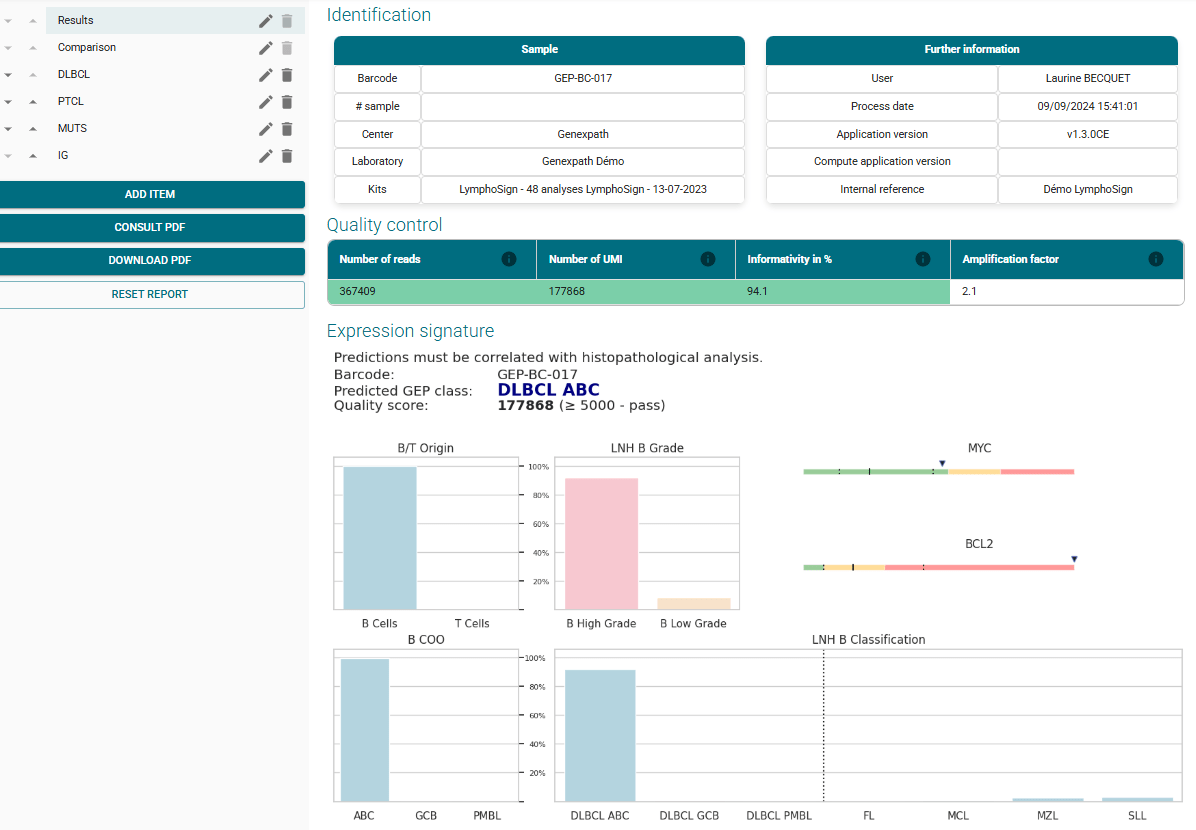
Results obtained
Access to LymphoSign's documentation
Why are you asking me those information ?
By providing this information, you agree to be contacted so that we can best respond to your requests. This allows us to provide you with additional personalized information. Your data will be deleted upon simple request.
Publications related to LymphoSign
-
Determination of Molecular Subtypes of Diffuse Large B-Cell Lymphoma Using a Reverse Transcriptase Multiplex Ligation-Dependent Probe Amplification Classifier: A CALYM Study
PMID: 29054399
-
Biological and Clinical Relevance of Associated Genomic Alterations
PMID: 27923841
-
Oncogenic events rather than antigen selection pressure
PMID: 27737507
-
Next-Generation Sequencing
PMID: 26819451
-
Accurate Classification of Germinal Center B-Cell-Like/Activated B-Cell-Like Diffuse Large B-Cell Lymphoma Using a Simple and Rapid Reverse Transcriptase-Multiplex Ligation-Dependent Probe Amplification Assay: A CALYM Study
PMID: 25891505