
Test SarcomaFusion – CE IVD
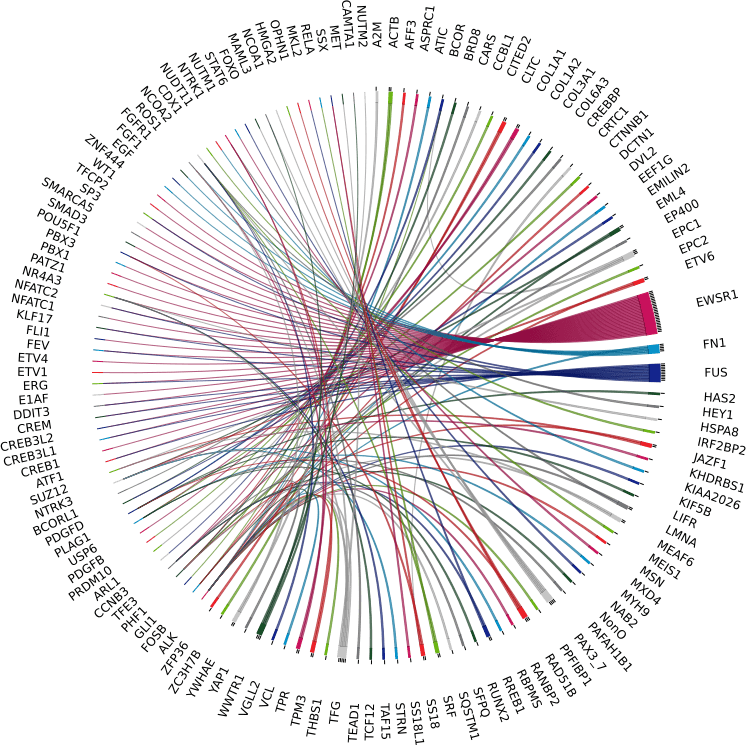
SarcomaFusion: detect more than 140 fusion transcripts identified in sarcomas in a single test
Fusion transcripts are present/found in approximately 1/3 of soft tissue tumors. To date, the literature has described more than 140 fusions. They are often associated with a particular histological subtype and consequently with specific treatments. They therefore constitute ideal molecular diagnostic markers.
The detection of these fusion transcripts is therefore essential for the management of the patient. The SarcomaFusion test allows the detection of fusion transcripts identified in sarcomas in a single test
The SarcomaFusion test is a cost-effective solution for detecting fusion transcripts identified as responsible for sarcomas.
A simple and rapid protocol for pathologists and molecular biologists
A unique test, based on a patented ligation-dependent PCR technology, allows the pathologist or molecular biologist to detect fusion transcripts, from a fresh, frozen or paraffin-embedded sample, among 140 possible, and only in 1 reaction.
The simplicity of the protocol test makes it possible to obtain the results of the analysis from the tumor RNA in less than 24 hours.
Since the amount of tumoral RNA required is very small, a needle biopsy is sufficient to obtain results.
The SarcomaFusion test is extremely robust and sensitive.

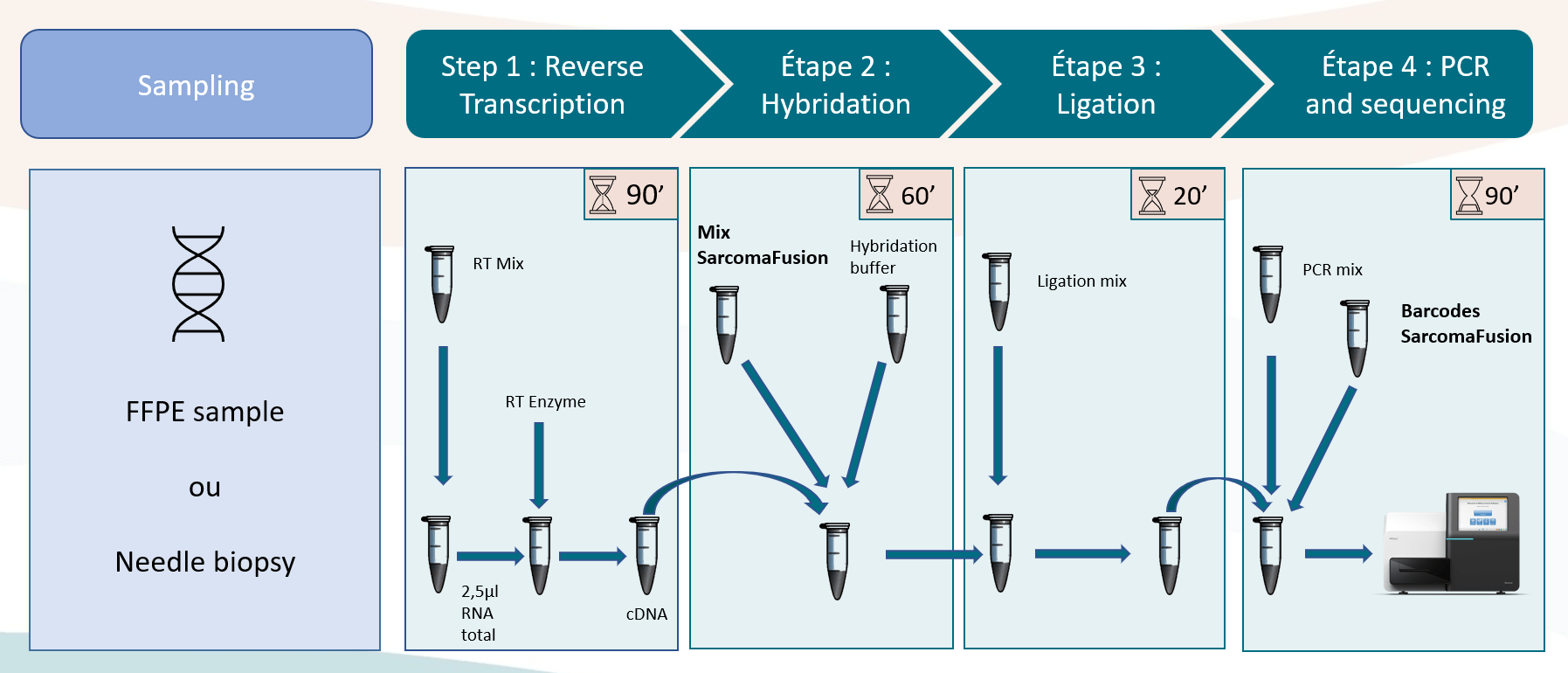
A simple and rapid protocol
The In Vitro test consists of 4 steps with a total duration of approximately 1/2 day including 2h to 2h30 hands-on.
NGS sequencing of the LymphoSign test requires only 100,000 reads per sample.
SarcomaFusion can be sequenced with other libraries and the barcodes are provided with the kit.
A fine computer analysis
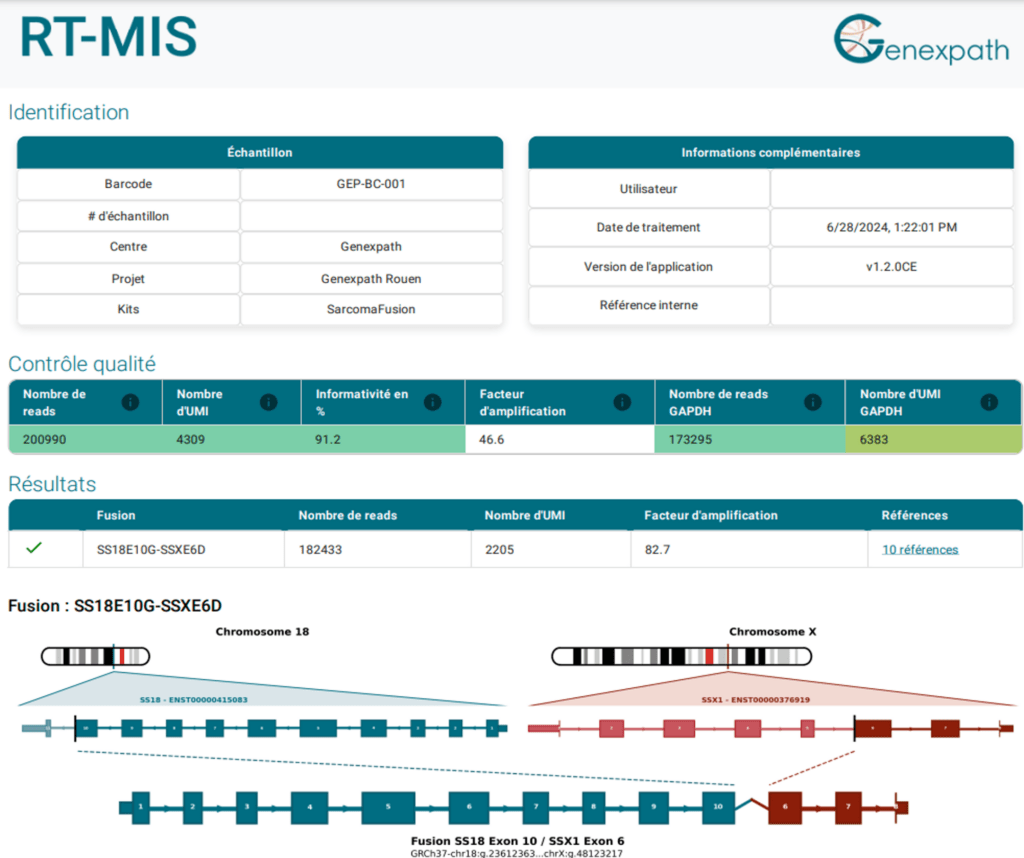
After sequencing, the RT-MIS online analysis platform allows automated analysis of FASTQ files.
It provides the user with the list of any fusions detected in the biological sample as well as the associated quantitative information.
RT-MIS also provides the bibliography relating to these fusions.
The RT-MIS use is simple, fast and secure for the user.
The SarcomaFusion test is CE-IVD marked. Consult the publication of reference

